About NeoCore
NeoCore has gradually become the benchmark of biochip industry in the country since its establishment.
NeoCore has developed multiple advanced testing technologies and related instruments, and led a trend in highly sensitive and highly specific molecular testing to improve the biotechnology standards and promote internationally.
Since its establishment more than 40 years ago, we have been committed to a green energy environment and medical care. We have the mastery and professional knowledge of raw materials and insist on the quality and service of medical equipment. We can do it and achieve excellence.




Leading Technology and Excellent Service
NeoCore has been upholding the spirit of serving customers and integrating cutting-edge technology since founded in 1998. NeoCore has been providing our customers with reliable and high-quality products at reasonable prices by carrying out our utmost commitment of implementing quality assurance and customer-focused policies, accurate validation inspections, customer orientation as well as continuous quality improvement.
Technology Innovation and Precise Inspection
NeoCore’s manufacturing factory is located in
Zhunan Science Park. It is recognized by the Quality
Management System (QMS) and ISO13485. In terms
of corporation operations, the company has
successively launched machine upgrades,
automation equipment integration and new
business layout in order to sustainably optimize its
performance and carry out diversified operations.
The current technology platform is not only applied
to the essence of clinical testing, but also improves
the timeliness problem of traditional testing
methods to further increase accuracy of achieving
the very definition of “early detection and timely
treatment” and meeting market demand.
NeoCore’s Commitment
Consistent and comprehensive solution.
Implement Quality
Strictly control the quality of products and services
Accurate Inspection
Ensure inspection accuracy
Customer Oriented
Meet customer needs and services
Quality Improvement
Continuous advancement of technology
Company Base

Taiwan Headquarters
9F-8, No. 395, Sec. 1, Linsen Rd., East Dist., Tainan City 701024, Taiwan (R.O.C.)
Science Park Branch
No. 31, Kezhong Rd., Zhunan Township, Miaoli County 35053, Taiwan (R.O.C.) (Zhunan Science Park)
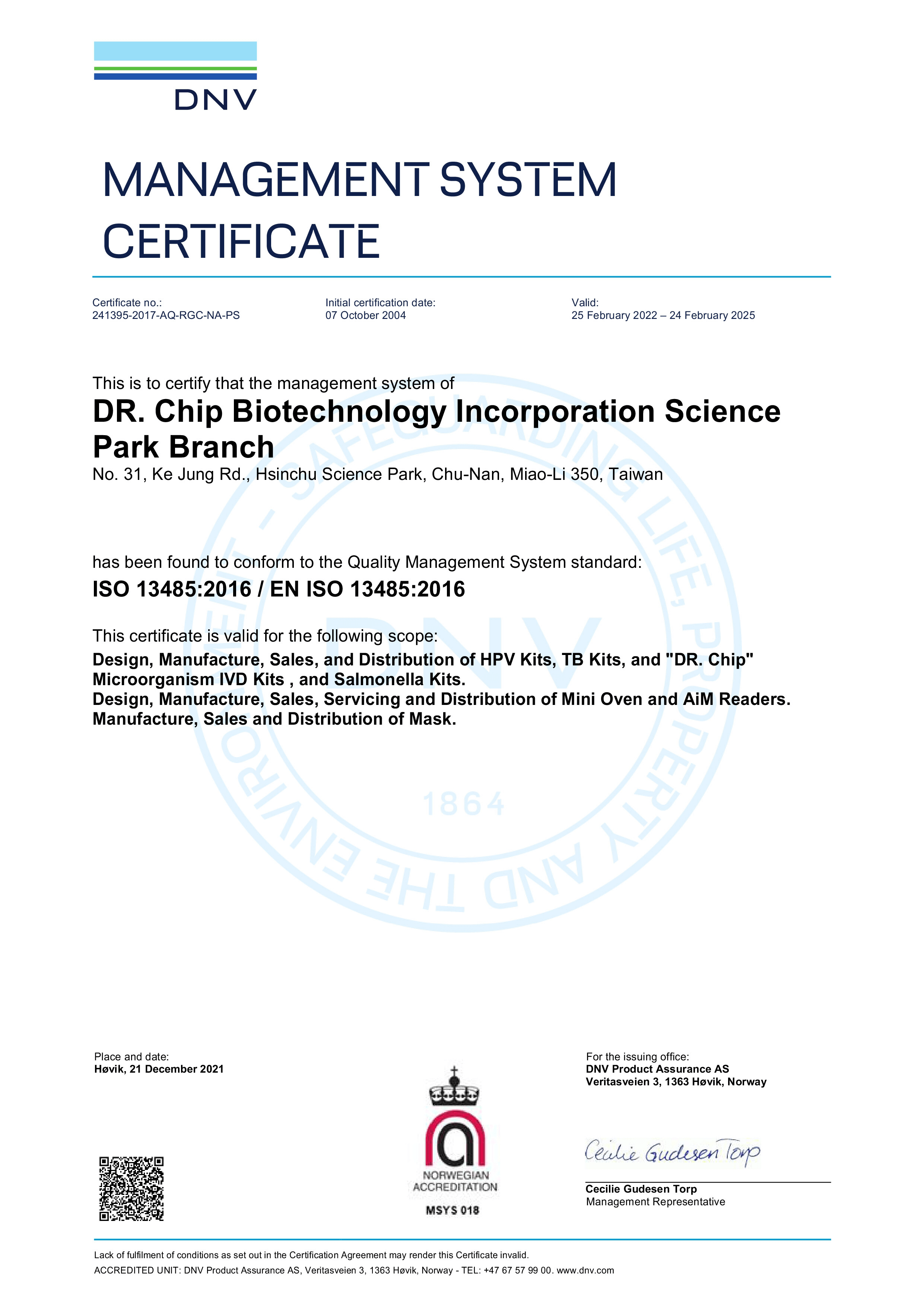
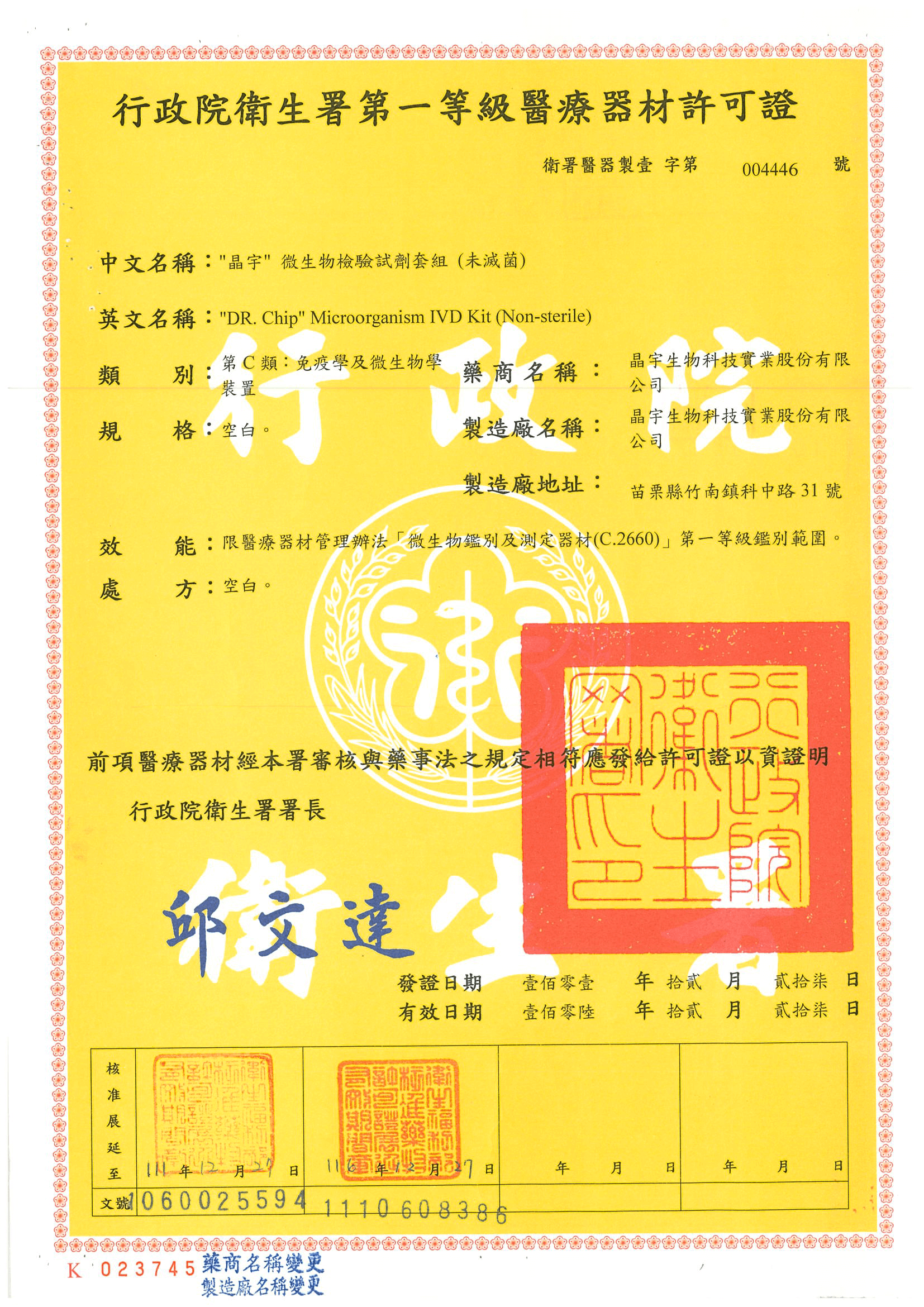
Certification
Purchase with confidence only by obtaining the medical equipment license from the Ministry of Health and Welfare.
Heading #2 | |
|---|---|
2004 |
|
2009 |
|
2015 |
|